HR+, HER2- Early Breast Cancer
Interpreting Adjuvant Breast Cancer Clinical Trials
Patients with early breast cancer (EBC) are treated with curative intent1
Defining surrogate endpoints
To observe efficacy outcomes for patients with EBC, it has become essential to define surrogate endpoints for OS. This is especially crucial in the adjuvant setting.5 To ensure the use of standardized endpoints, careful consideration should be taken across the clinical trial life cycle.5




Invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS) are surrogate endpoints for OS
Recurrence of breast cancer can be local, regional, or distant.6 Risk of recurrence peaks early in patients at ~2 years after primary diagnosis.6,7 IDFS is a composite endpoint that includes local, regional, and distant recurrence. DRFS emphasizes distant recurrence or metastasis in a vital organ.5,8
Local recurrence is in the breast. Regional recurrence is in the lymph nodes. Examples of distant recurrences are in the brain, lung, liver, or bone.
IDFS and DRFS: composite endpoints used in adjuvant breast cancer clinical trials
Both IDFS and DRFS are standardized, clinically meaningful endpoints in adjuvant breast cancer clinical trials.2,5
- IDFS provides information on local invasive recurrence, regional invasive recurrence, distant recurrence, death of any cause, invasive ipsilateral breast tumor recurrence, invasive contralateral breast cancer, and second primary nonbreast invasive cancer
- DRFS provides information on distant recurrence and death of any cause
- Ductal carcinoma in situ (DCIS) includes both ipsilateral and contralateral DCIS
- IDFS Endpoint
- Local invasive recurrence: yes
- Regional invasive recurrence: yes
- Distant recurrence: yes
- Death of any cause: yes
- Invasive ipsilateral breast tumor recurrence: yes
- Invasive contralateral breast cancer: yes
- DCIS: no
- Second primary non-breast invasive cancer: yes
- DRFS Endpoint
- Local invasive recurrence: no
- Regional invasive recurrence: no
- Distant recurrence: yes
- Death of any cause: yes
- Invasive ipsilateral breast tumor recurrence: no
- Invasive contralateral breast cancer: no
- DCIS: no
- Second primary non-breast invasive cancer: no
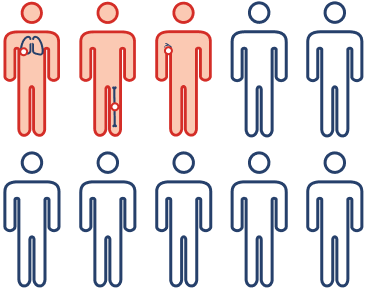
Implications for patients with high-risk disease
Because ~30% of patients with high-risk, HR+, HER2- EBC may experience disease recurrence in 5 years,8 IDFS and DRFS are very meaningful clinical endpoints. Improvement in IDFS or DRFS means fewer recurrences and fewer instances of incurable metastatic disease, which are critically important in practice.

Understanding the Carryover Effect of Oral Endocrine Therapy
Adjuvant endocrine therapy (ET), such as tamoxifen and aromatase inhibitors (AIs), is the current standard of care for patients with hormone receptor–positive (HR+) EBC.9-11 Carryover effect is the term used to describe the long-lasting benefit of ETs in reducing the risk of recurrence after stopping the initial treatment.12,13


5 years of adjuvant tamoxifen:
- Significantly lowered disease recurrence throughout the first 15 years (recurrence ratio [RR], 0.61; standard error [SE], 0.03)
- Reduced mortality by 30% throughout the first 15 years (RR, 0.77; SE, 0.05)


Extending adjuvant tamoxifen to 10 years:
- Continued to lower disease recurrence (RR, 0.90; 95% confidence interval [CI], 0.79-1.02) and mortality (RR, 0.97; 95% CI, 0.79-1.18) during years 5-9
- Reduced the risk of recurrence (RR, 0.75; CI, 0.62-0.90) and mortality (RR, 0.71; 95% CI, 0.58-0.88) after year 10
AIs also have a carryover effect
In patients with ER+ disease, 5 years of adjuvant AI when compared with no ET17:
- Lowered disease recurrence by two-thirds during treatment and by one-third during years 5-9 following treatment and reduced breast cancer mortality by 40% throughout the first 10 years

Recommended therapy duration
A minimum of 5 years of ET is recommended for women with stage 1-3 ER+ EBC. Up to 10 years of extended therapy is recommended for women with higher-risk, node-positive disease.

Understanding the carryover effect
As healthcare providers, it is important to understand the carryover effect of ET for patients with HR+ EBC and how treatment duration, ET adherence, and choice of therapy may influence these effects.
Developing an individualized treatment plan for each patient can help to optimize patient care and reduce risk of disease recurrence.
Related Resources
Downloadable PDFs
INFOGRAPHIC: High-Risk HR+, HER2- EBC: Carryover Effect of Oral Endocrine Therapy (PDF)
IDFS: a clinically meaningful endpoint in adjuvant breast cancer clinical trials
Watch Dr. O’Shaughnessy describe why Invasive Disease-Free Survival (IDFS) is an important clinical trial endpoint used in high-risk, HR+, HER2- early breast cancer studies.
Defining DRFS as an endpoint in adjuvant breast cancer clinical trials
Dr. O’Shaughnessy defines distant relapse-free survival (DRFS) explains why it is an important endpoint in high-risk HR+, HER2- early breast cancer trials.
References
- Yung R, et al. Breast Cancer Res Treat. 2020;180(3):747-757.
- Gourgou-Bourgade S, et al. Ann Oncol. 2015;26(5):873-879.
- Garutti M, et al. Cancers. 2022;14(1898):1-17.
- Giaquinto AN, et al. CA Cancer J Clin. 2022;72(6):524-541.
- Hudis CA, et al. J Clin Oncol. 2007;25(15):2127-2132.
- Colleoni M, et al. J Clin Oncol. 2016;34(9):927-935.
- Cheng L, et al. Cancer Epidemiol Biomarkers Prev. 2012;21(5):800-809.
- Sheffield KM, et al. Future Oncol. 2022;18(21): 2667-2682.
- Cardoso F, et al. Ann Oncol. 2019;30(8):1194-1220.
- Korde LA, et al. J Clin Oncol. 2021;39(13):1485-1505.
- Burstein HJ, et al. Ann Oncol. 2021;32(10):1216-1235.
- Chumsri S, Thompson EA. Lancet. 2020;395(10218):91-92.
- EBCTCG. Lancet. 2005;365(9472):1687-1717.
- EBCTCG. Lancet. 2011;378(9793):771-784.
- Davies C, et al. Lancet. 2013;381(9869):805-816.
- Burstein HJ, et al. J Clinic Oncol. 2019; 37(5):423-438.
- EBCTCG. Lancet. 2015;386(10001):1341-1352.
VV-MED-154879