Implementing Biomarker-Informed Cancer Care in NSCLC
Non-Small Cell Lung Cancer
Most cases (47.6%) of non–small cell lung cancer (NSCLC) cases are advanced or metastatic at diagnosis,1 and 54% of early-stage cases will eventually progress to stage IV.2
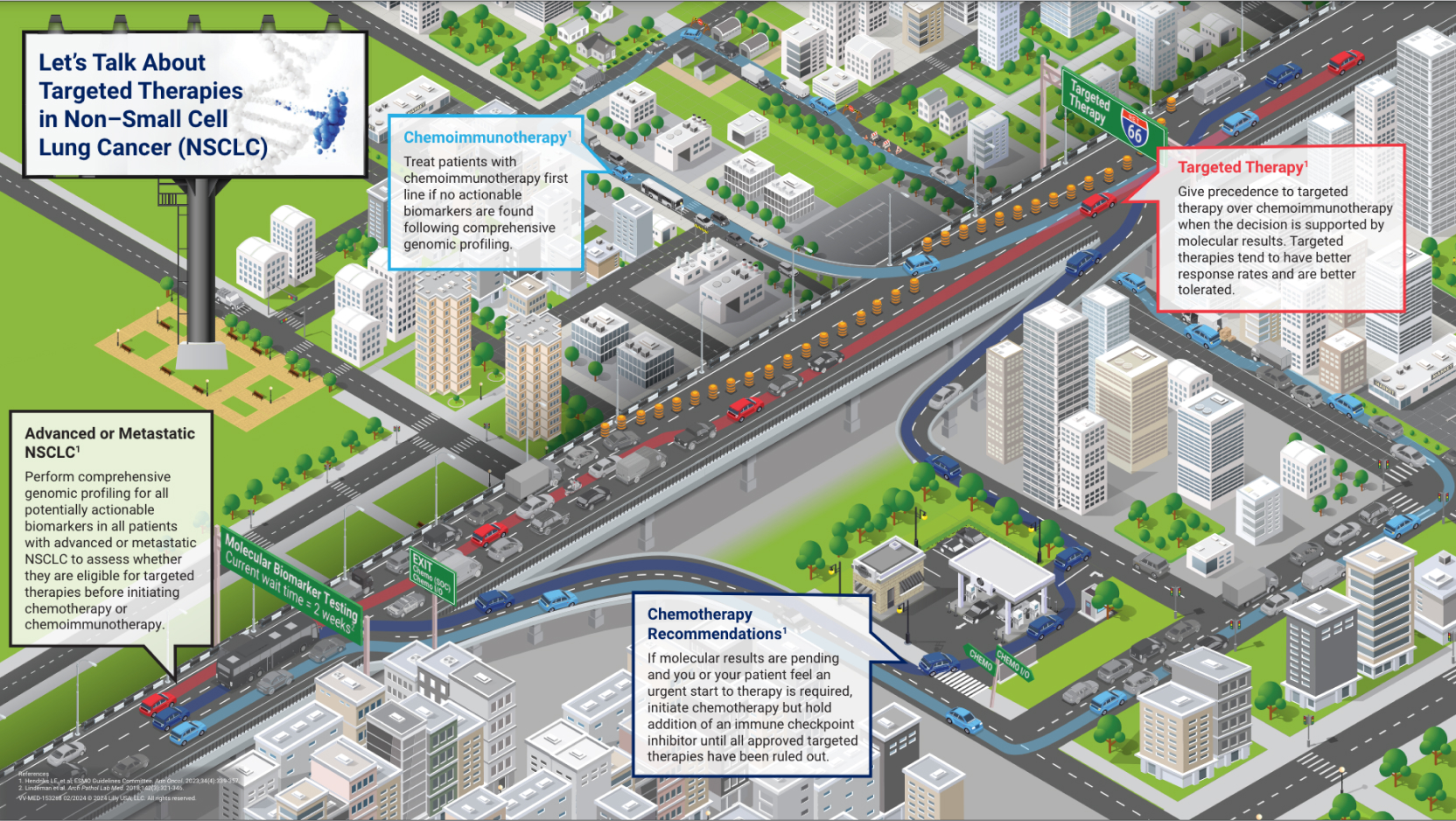
Patients with advanced or metastatic NSCLC may be eligible to receive targeted therapy and should have comprehensive genomic profiling performed to test for actionable biomarkers.3
However, less than 40% of eligible patients with NSCLC receive targeted therapy.4 Practice gaps in the NSCLC treatment journey contribute to this patient loss.4 Implementing strategic solutions to standardize and optimize protocols in key workflow areas may help improve overall patient access to targeted therapies. Click below to learn more about each step of the NSCLC treatment journey.

Patient Identification


Identifying patients who will benefit from precision medicine and selecting the right first-line therapy is essential to optimizing patient outcomes in NSCLC.5,6 Inappropriate first-line therapies based on incomplete testing are unlikely to be effective and can lead to increased toxicity with subsequent targeted treatments. To optimize selection of the most appropriate first-line therapy, comprehensive molecular testing, in combination with conservative use of IHC, should be performed at diagnosis before initiating treatment in all patients with advanced or metastatic NSCLC.
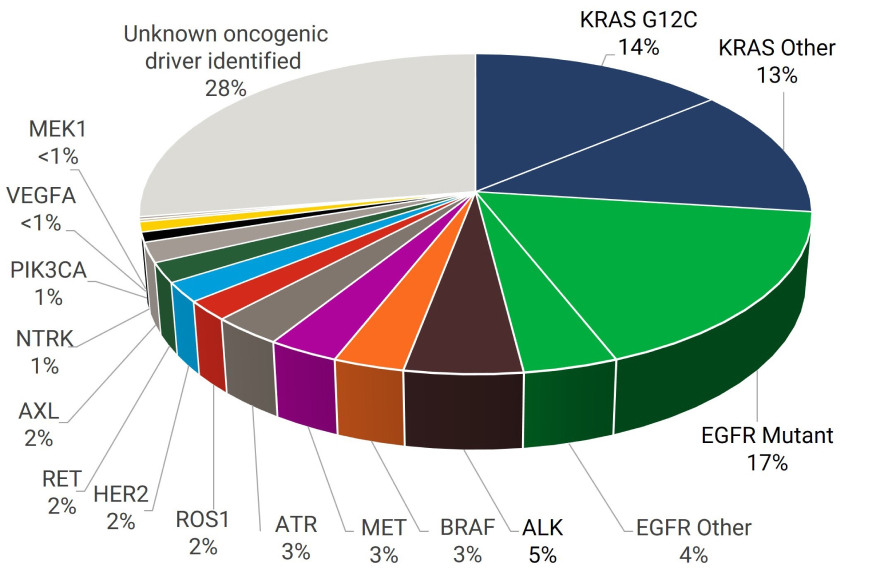
More than half of all patients with NSCLC harbor potentially actionable genomic alterations; however, many of these individual alterations occur with relatively low frequency.7 Thus, identifying patients who will benefit from precision medicine often involves detecting relatively rare gene alterations. Successfully doing so allows for targeting of actionable alterations with precision medicine, which potentially improves survival in patients with NSCLC.5,6
The use of targeted therapy has been associated with a more favorable outcome in advanced NSCLC, with a 31% reduction in risk of death and improved survival duration that was approximately 1.5-fold longer compared with patients with an identified mutational driver but did not receive targeted therapy.8 Comprehensive testing is recommended to identify potentially actionable biomarkers and optimize choice of therapy in patients with NSCLC.9
Frequency of Genomic Alterations in NSCLC Adenocarcinoma1

PD-1 and PD-L1 Testing

Microsatellite Instability and DNA Mismatch Repair

Tumor Mutational Burden
Sample Collection


Collection technique affects diagnostic yield and downstream success of genomic testing (molecular yield). Identifying the reason for biopsy can help choose the best method to optimize yield.71-73
Thoughtful selection of the appropriate biopsy approach (eg, technique, needle gauge) for specimen collection can ensure sufficient material is available to render a diagnosis and provide comprehensive biomarker testing from a single procedure.74
If initial biopsy reveals lung adenocarcinoma, but limited tissue remains after diagnostic workup75:
- Communicate presence of limited testing material to the ordering provider
- Prioritize testing for targets most likely to be identified and/or smaller panels that are less likely to be QNS
- Consider ordering a liquid biopsy (ctDNA assay)
- Consider repeat biopsy, and communicate “molecular priority” protocol for known diagnosis
- Consider testing alternative specimens (see “Tissue Stewardship”)
Approximate Diagnostic Yield of Tissue Collection Techniques71-73

ROSE has the potential to positively impact outcomes.76-80

Each molecular laboratory will have a minimal amount and concentration of tumor cells required for accurate detection of molecular alterations, based on the specific tumor enrichment protocols available and assay platforms used for testing.81 Most NGS platforms require a sample size of ≥25 mm² tumor surface area and ≥20% viable tumor nuclei per sample.82,
Tissue blocks that are adequate for molecular testing should be tracked and by flagging in the report for the tissue navigator and/or lab technician.84
Tissue Stewardship


While resection specimens have abundant tissue available for testing, they are available only in a small subset of patients with NSCLC.81 Thus, it is necessary to consider other types of specimens that might require testing.81 The majority of patients with lung cancer are diagnosed after examination of a small biopsy or cytology specimen of the primary lesion or a suspected metastasis.81 It has been well documented that small biopsy specimens, cytology cell blocks, and cytology touch imprints or aspirate slides are suitable for molecular testing.81
Impact of Acquisition Method on Sequencing Success85


Implementing standardized tissue-specific procedures and optimizing pre-analytic conditions by processing samples according to lab specifications via a preferred vendor may help reduce quantity not sufficient (QNS) rates and downstream sequencing failure.85,87

Selecting a Molecular Test


Although a single gene test (SGT) may have a shorter turnaround time than NGS, NGS is faster than sequential testing with SGTs.88 Upfront NGS testing in metastatic NSCLC is associated with reduced cost and time-to-results for commercial and CMS payers.89
Additionally, with a sequential single-gene approach, tumor tissue may be insufficient, and some biomarkers may come back without results, which may impact treatment strategy and patient outcomes.86
Single Gene Test vs Comprehensive Profiling86

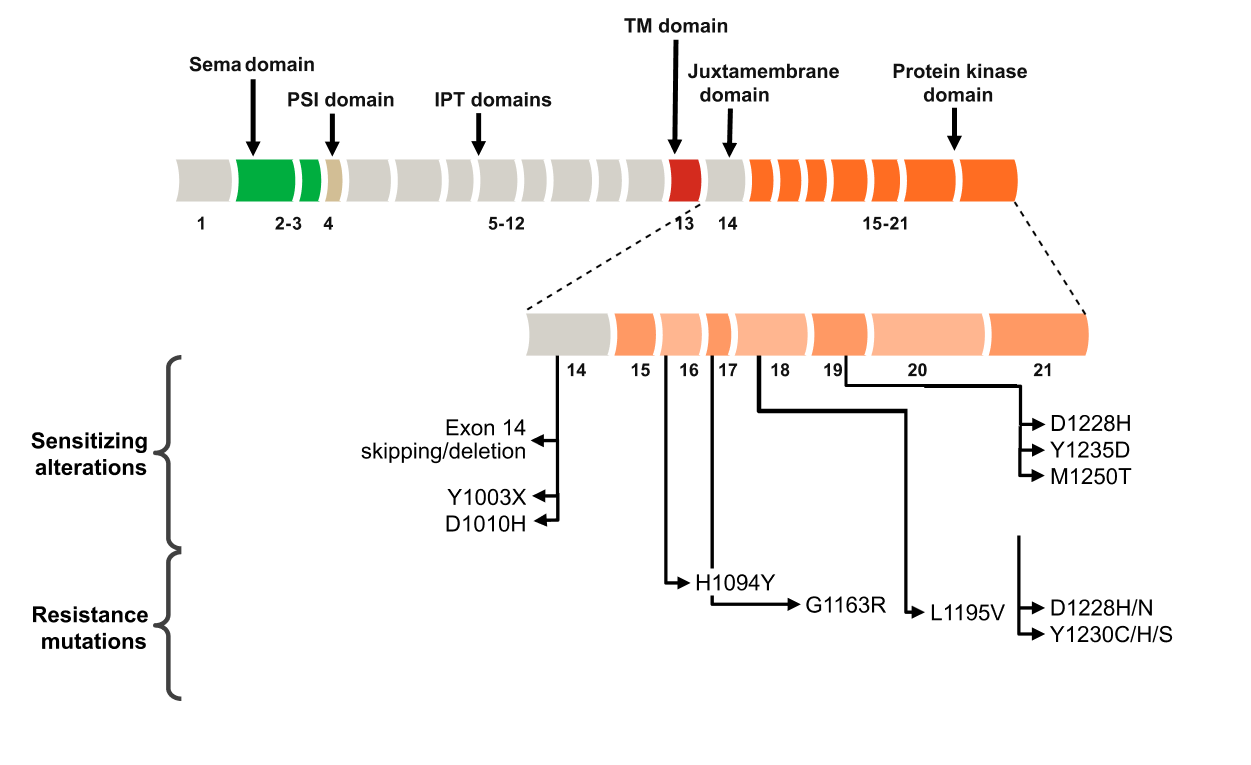
Molecular Testing Options to Identify Targetable, Sensitizing Alterations in NSCLC9,14,47,55,68,90-94

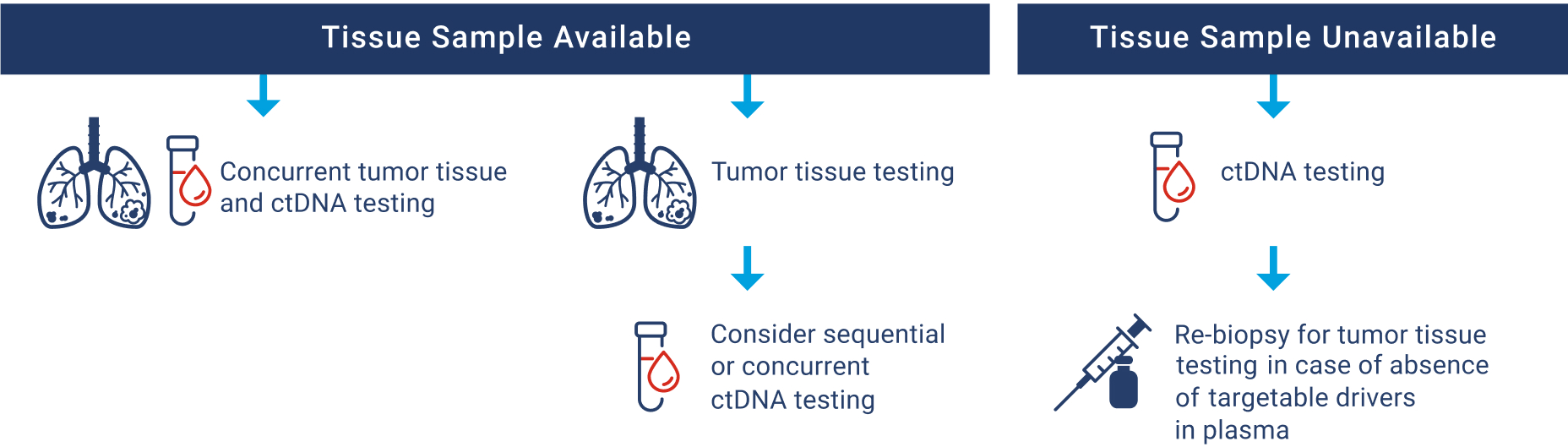
Use of tissue- and plasma-based (ctDNA) testing are acceptable in the advanced/metastatic NSCLC setting, whether used sequentially or concurrently. Concurrent testing has the potential to reduce TAT if a protracted wait is anticipated for tissue-based results or may also be indicated in cases where there is limited tumor tissue available and QNS is probable. The identification of an actionable driver mutation by either method is sufficient to start therapy. Conversely, the absence of an actionable driver mutation in either assay does warrant the use of a complementary method. Additional advantages and disadvantages to both testing approaches are outlined below.

Disadvantages: Longer TAT, limited tissue quantity/quality, invasive at disease progression, re-biopsy not always feasible, tumor heterogeneity

Disadvantages: Non-DNA biomarkers are not evaluable, Low tumor fraction, presence of mutations from sites other than the target lesion, most commonly ChIP

Disadvantages: Potential increased costs
Diagnostic Algorithm for ctDNA Testing Use in Treatment-naïve Advanced/Metastatic NSCLC89,95
Multidisciplinary Communication



Learn from Drs. Jill Kolesar and Ravneet Thind about how the multidisciplinary communication facilitated by a molecular tumor board can enable biomarker-informed decision-making and optimize therapeutic options for patients.
As molecular diagnostic testing is becoming increasingly sophisticated and complex, molecular tumor boards can bring relevant expertise to this rapidly emerging field and are crucial for patient care.

>16% increase in recommended treatment choice96,97

~30% decrease in time to treatment initiation96-98

~40% absolute increase in overall patient survival rate99
Expanding Molecular Tumor Boards to Community Settings with Drs Jill Kolesar and Ravneet Thind
QUESTION 02: What are the advantages of an MTB?
Dr. Kolesar: We implemented a molecular tumor board with the intention of increasing the rates of molecular testing, which we anticipated would decrease turnaround time for testing, increase access to clinical trials, and ultimately, more patients receiving biomarker-informed treatment decisions.
Dr. Thind: Due to the general cytotoxicity of conventional chemotherapy, focus has now shifted to novel therapeutic targets that can be exploited based on genomic data and molecular tumor board recommendations. So as Jill mentioned previously, molecular tumor boards can increase guideline-concordant testing and decrease turnaround time for molecular testing, which enables biomarker-informed decision-making and optimizes therapeutic options for our patients.
Dr. Kolesar: Dr. Thind, as you can see in this figure from our case-control study, we showed that patients with non–small cell lung cancer who had their cases reviewed by a molecular tumor board had improved overall survival compared to those who did not, regardless of whether they were treated in an academic setting or a community.
- Hosting virtual meetings
- Providing a molecular tumor board-dedicated navigator
Expanding Molecular Tumor Boards to Community Settings with Drs Jill Kolesar and Ravneet Thind
QUESTION 02: What elements were necessary for extension of the MTB to rural community sites?
Dr. Kolesar: I think the most important part was really to make it easy for sites to participate. So to do that, we held a virtual meeting, and we had a molecular tumor board coordinator that managed the cases referred from the community sites.
Our coordinator interacts with clinic staff from the treating physician office to gather up the notes and NGS reports for review and to provide the written recommendations back. They also coordinate the virtual meeting.
Dr. Thind: Yes, I agree. I think having a molecular tumor board–dedicated navigator has been a game changer. Along with excellent communication and support from the University of Kentucky, with clear and precise explanation of the entire process.
Dr. Kolesar: I think it was also really helpful to demonstrate the clinical benefit showing that patients with molecular tumor board review had better outcomes.
And having wide clinical and genomic expertise, with evidence grading were also critical to the success of the molecular tumor board.
- Consolidation of reports and upload into EMR
- Follow-up of results and communication of findings
- Facilitation of multidisciplinary discussions

Treatment Decision


- Test for certain molecular and immune biomarkers in all appropriate patients with NSCLC
- Have molecular testing results for the actionable oncogenic mutations before starting systemic therapy combined with immunotherapy
- Treat patients with oncogenic driver mutations with targeted first-line therapy for that oncogene rather than first-line ICIs
Welcome everybody. My name is Tim Burns, and I am a principal investigator and thoracic medical oncologist at the University of Pittsburgh Medical Center, Hillman Cancer Center. My clinical and translational research interests focus on the development of novel targeted therapy approaches for oncogene-driven lung cancer.
Today, we’ll be discussing the case of a patient who was referred into my care. The patient was a 73-year-old female with stage IVa non–small cell lung cancer. At diagnosis, her adenocarcinoma of the lung had begun to spread to the lymph nodes, and it caused a right pleural effusion. When I met this patient, unfortunately, she had been admitted into the cardiac intensive care unit for florid new onset of congestive heart failure. Now, we’ll review the patient’s treatment history leading up to this point and after she was transferred into my care.
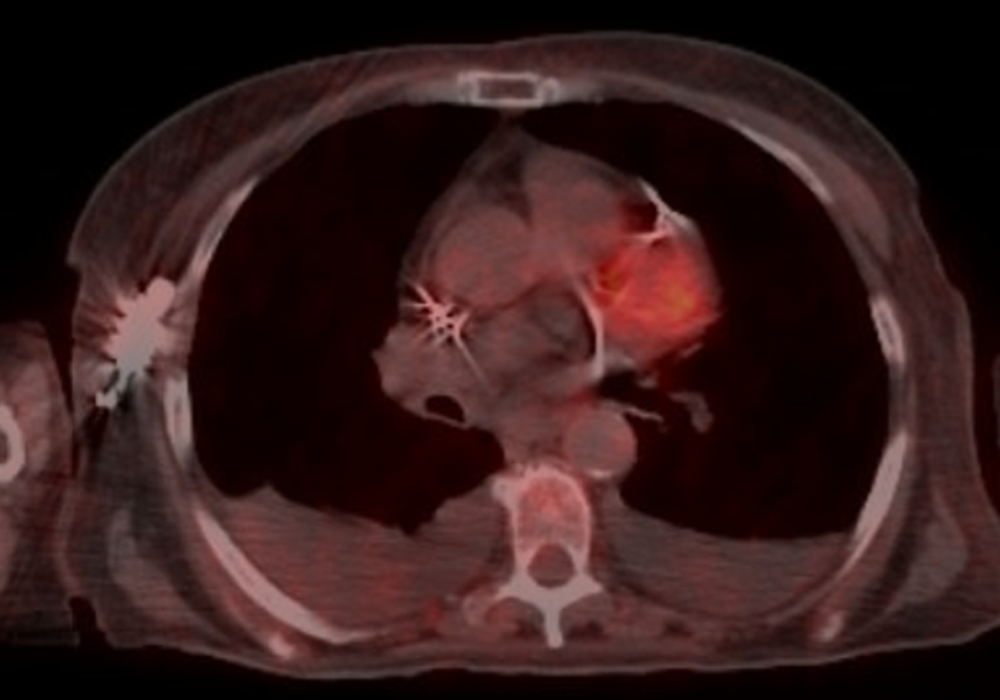
The patient was a never smoker and denied any drug or alcohol use. She had a history of asthma, and in the 6 months leading up to her diagnosis, she had been treated for 3 occurrences of pneumonia without significant improvement. She eventually presented to the emergency room with persistent cough and dyspnea. A CT scan at that time found a right middle lobe, right lower lobe mass with mediastinal adenopathy. A subsequent PET/CT scan revealed a PET-avid 7 cm right upper lobe and middle lobe mass-like structure, supraclavicular and large mediastinal lymphadenopathy, and a loculated right pleural effusion.
The patient underwent a bronchoscopy, EBUS, and biopsy of the mass and lymph nodes at stations 4, 7, and 10. Pathology results were consistent with adenocarcinoma of the lung. Tissue samples were also sent for comprehensive genomic profiling and immunohistochemistry, which revealed a PD-L1 of 70%, a KIF5B-RET fusion, a PIK3CA mutation, and a tumor mutation burden of 6.9 mutations/Mb.
So, in this patient, there are several potential targetable molecular biomarkers that we found. First, the patient has a targetable RET fusion in her tumor, and there are first-line targeted therapies with selective RET TKIs, which has been shown to be superior both in response rate and progression-free survival compared to chemotherapy. And so clearly the choice for this patient would be to treat the RET fusion with a selective RET inhibitor. You also see a PIK3CA mutation. In lung cancer, although there are PIK3CA inhibitors that are approved for breast cancer; there are none that are approved in lung cancer. And to date, there really has not been a lot of activity of these inhibitors in lung cancer. In addition, you'll notice there's a high PD-L1 expression here. Well, it's often the case when you have an oncogenic driver that you'll see high levels of PD-L1. And that's actually because a lot of these oncogenic drivers will drive PD-L1 expression. However, in that case, this high expression is unrelated to whether these tumors can respond to immunotherapy, and that should not be used as a way of selecting patients who respond to immunotherapy.
Despite what I just said on the last slide, this patient was initially cared for at an outside institution, and they were given chemoimmunotherapy in the first-line setting. She received 2 cycles of therapy until, unfortunately, she presented to the emergency room, now with right arm pain and swelling and was found to have new arterial thrombosis requiring an embolectomy and also had a DVT. She also had a CT scan at that time, which now revealed worsening right lower lobe opacification, new and enlarged left lobe nodules, and a right pleural effusion, which had increased and would now require a thoracentesis.
After discharge, she received 1 final cycle of Pembrolizumab alone. Two weeks following this treatment, she was admitted into the cardiac intensive care unit with florid new onset congestive heart failure and required a left ventricular assist device and a pacemaker for now complete heart block. A transthoracic echocardiogram revealed an ejection fraction of only 10%-15%. A right heart catheterization and biopsy confirmed autoimmune myocarditis. This patient was aggressively treated with immunosuppressive therapy, and luckily her ejection fraction improved to 40%-45%. A CT scan of the chest during this admission demonstrated a modest response to therapy in the mediastinal adenopathy and right-sided consolidation; however, she had an increase in her right pleural effusion.
So, at this point we have a patient that's clearly progressing and has had serious side effects from her chemoimmunotherapy. She has required aggressive care for her autoimmune myocarditis, and at this point any further immunotherapy would be absolutely contraindicated. If this patient were to be rechallenged with immunotherapy, it is likely that she would potentially die from her myocarditis if she had another attack. Given that chemoimmunotherapy is no longer an option, the question is what should we do next for this patient. And I think this is, as we talked about before, we have 2 FDA-approved RET-specific inhibitors that we could use for this patient. And I think that would be a reasonable approach.
After discharge, the patient followed up in our clinic to discuss next steps in her therapy. She was now on anticoagulation for her recent clots and on medication both for her heart failure and steroid-induced diabetes. Despite this, she had been feeling well since discharge, with some rare lightheadedness but no new or concerning symptoms. She was started on a selective RET inhibitor, and the patient was followed closely with visits every 2 weeks for side effect monitoring, laboratory work, and EKG monitoring.
After 2 months on a selective RET inhibitor, the patient had her first restaging PET-CT, which revealed resolution of her mediastinal lymphadenopathy and improvement of her previous right-sided consolidation. Subsequent scans demonstrated deepening of this response without evidence of PET avidity.
The patient has tolerated therapy well with only intermittent nausea, which has not required antiemetic therapy, dose holding, or dose reduction. She continues on therapy almost 2 years later with continued response on radiographic imaging.
I think there are several key takeaways from this case. The first is that all of our patients in the metastatic setting and now even in earlier stages should be getting comprehensive genomic testing. And this is critical to identify these patients who can benefit from targeted therapy, such as in this case, and also select patients that will not benefit from immune checkpoint inhibitors. The advantage of this is that we're getting patients who potentially can have higher responses and durable responses. If you have a patient where you can't wait for the testing to come back, that's fine. But what I would recommend in those situations is to hold the immunotherapy for the first cycle and wait for that molecular testing to come back if you're worried about disease burden. There's a couple reasons for doing this. One is that if we give targeted therapy after immunotherapy, there can be a high side effect profile. And two, you may cause unnecessary autoimmune side effects like we saw in this patient. I want to thank everybody for listening to this patient case presentation. We hope the information provided here will have a positive impact on your treatment approach for patients with advanced or metastatic non–small cell lung cancer.
- Judicious use of IHC to conserve tumor tissue for molecular studies, especially in patients with advanced disease
- Broad molecular profiling using a validated test to minimize tissue use and potential wastage and assess all recommended biomarkers in all appropriate patients with NSCLC
- Patients with these oncogenic mutations should receive treatment with targeted agents
Welcome. My name is Luis Raez. I am the chief scientific officer and medical director of Memorial Cancer Institute at the Memorial Healthcare System. We are the third largest public healthcare system in the United States. We are situated in South Florida. Also, I'm still the Director of the Thoracic Oncology program. I am a lung cancer doctor by career and my focus of research is based on the molecular targets and liquid biopsies.
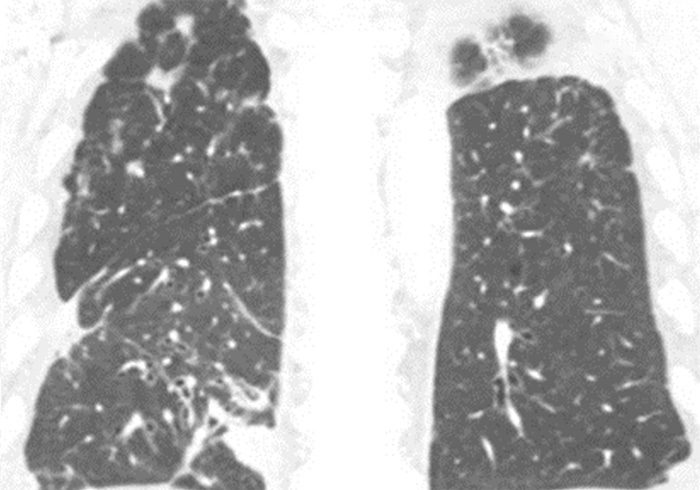
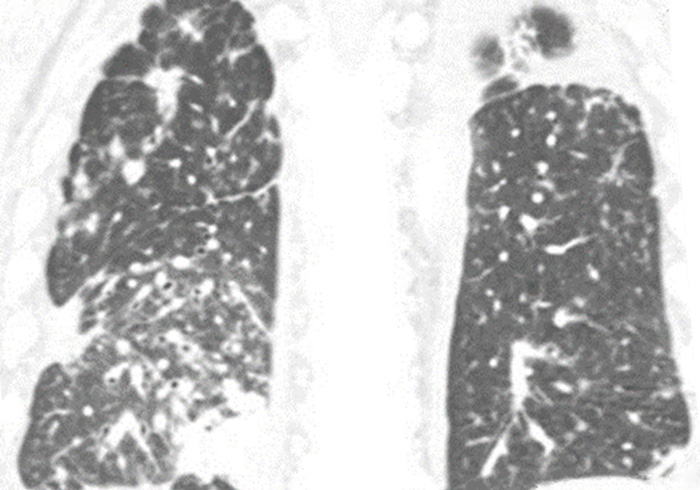
Today, we’ll be discussing the case of a patient who was referred to my care. The patient was an 80-year-old male with stage IV non–small cell lung cancer. At diagnosis, his adenocarcinoma was infiltrating both lungs, as you can see in the pictures, and had metastasized to his bones. Also, after 4 years of treatment after diagnosis, the patient was found to have developed brain metastases. Now we’ll review the patient’s story that brought him to this point as it was given to me when he transferred to my care.
The patient reported to never have smoked, used smokeless tobacco products, or partaken in alcohol or drug use. You can see in the table that the patient has some laboratory studies that we're displaying. The patient had weakness and malaise only as symptoms. So, the patient was still in good performance status and the patient was not very symptomatic as you can see. He was a senior citizen certainly, but he was very motivated looking for opportunities of therapy because as we can see later, he has failed several standard therapies.
The best molecular assessment probably can be done only with next-generation sequencing, because we know in metastatic non–small cell lung cancer, we have now 10 actionable genetic aberrations that need to be evaluated. So, there is no way nowadays that other technologies, like PCR, FISH, immunohistochemistry, can give us the answer because it's a matter of cost. It's a matter of time. That is why the only comprehensive or complete genetic analysis comes to doing next-generation sequencing in tissue or in blood or in both.
After his initial diagnosis of stage IVb non–small cell lung cancer, liquid and tissue samples were sent for molecular biomarker testing. The liquid biopsy results came back as negative for any actionable mutations or genetic aberrations. However, these results would not be confirmed by the tissue-based NGS test since the sample was deemed as “quantity not sufficient.” Immunohistochemistry of the tissue sample revealed low PD-L1 levels. Maybe some of us will have tried to pursue a little bit further and trying to get an answer from tissue because, as we said before, none of these technologies either liquid or tissue are 100% accurate yet, and that is why we need them to complement each other.
Because the patient was ruled to be ineligible for targeted therapies and immunotherapy was not part of the standard of care yet, he was started on palliative chemotherapy with Carboplatin and Paclitaxel, plus Denosumab for his bone metastases.
The best course of action at progression in this patient, today, probably will be to do another genetic analysis of the tumor, either by a liquid biopsy or a tissue biopsy, if possible. This patient was treated some years ago. That is what we will see in the next slides, the steps that he went for his therapy.
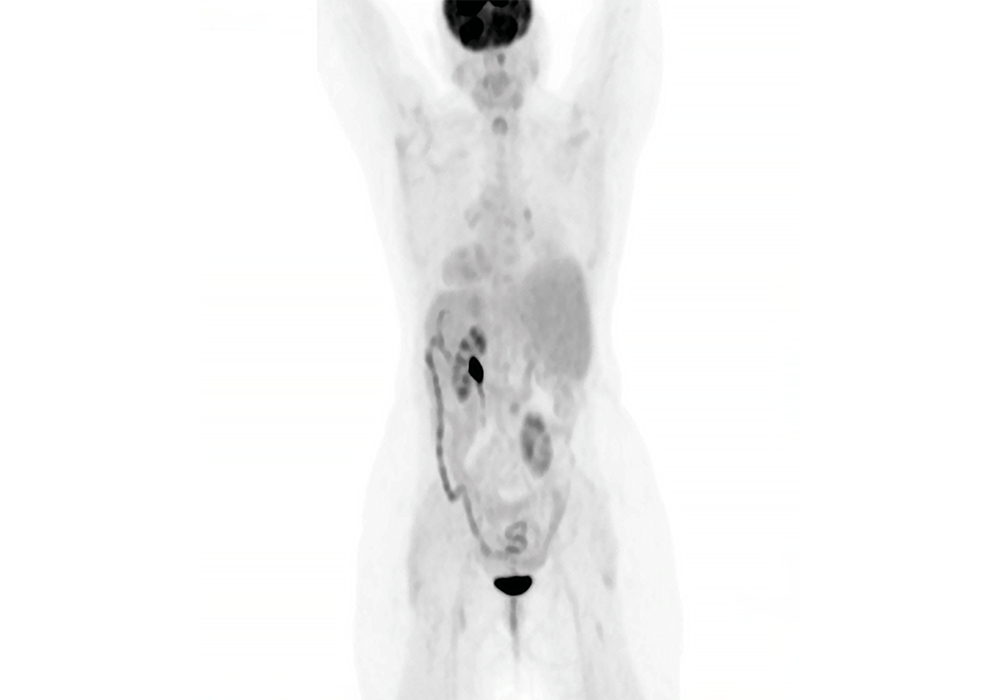
After the patient had disease progression with the frontline therapy, Nivolumab was added as a second-line therapy, at that time was the standard of care, until the patient had disease progression again. Over the next year-and-a-half to 2 years, the patient was cycled through 4 additional chemoimmunotherapy regimens as his disease continued to progress. It was on his last line of therapy, Gemcitabine, that brain metastases were found, and he was treated with whole brain therapy.
It was at this point that the patient was transferred into my care and his lung tumor was sent for tissue-based NGS testing, and the result came back positive for the most popular RET fusion, the KIF5B, which is an actionable biomarker in non–small cell lung cancer. And we had a clinical trial open for a RET inhibitor in our cancer center, because this was two years before any RET inhibitor approval, and we were able to enroll the patient in the clinical trial.
The patient came to us because he was running out of options. He already has tried 6 different lines of therapy including 5 chemotherapeutic agents and immunotherapy. So basically, for him the standard of care probably had only 1 or 2 more chemotherapeutic drugs available with a very low chance of success at that time in the standard of care.
At this point, the patient was started with a RET inhibitor at a conventional dose.
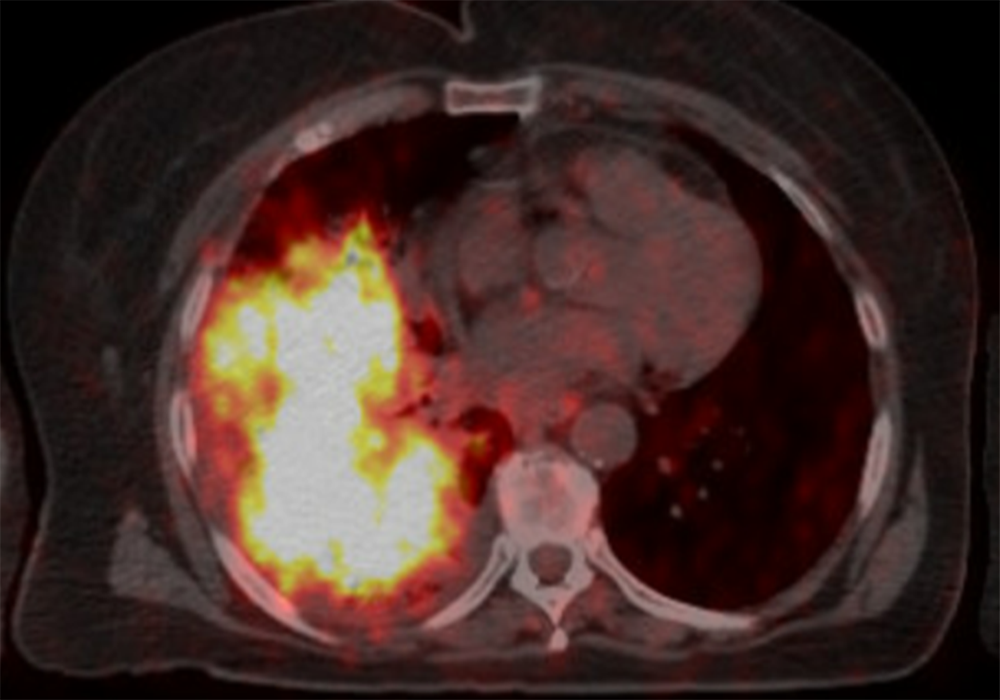
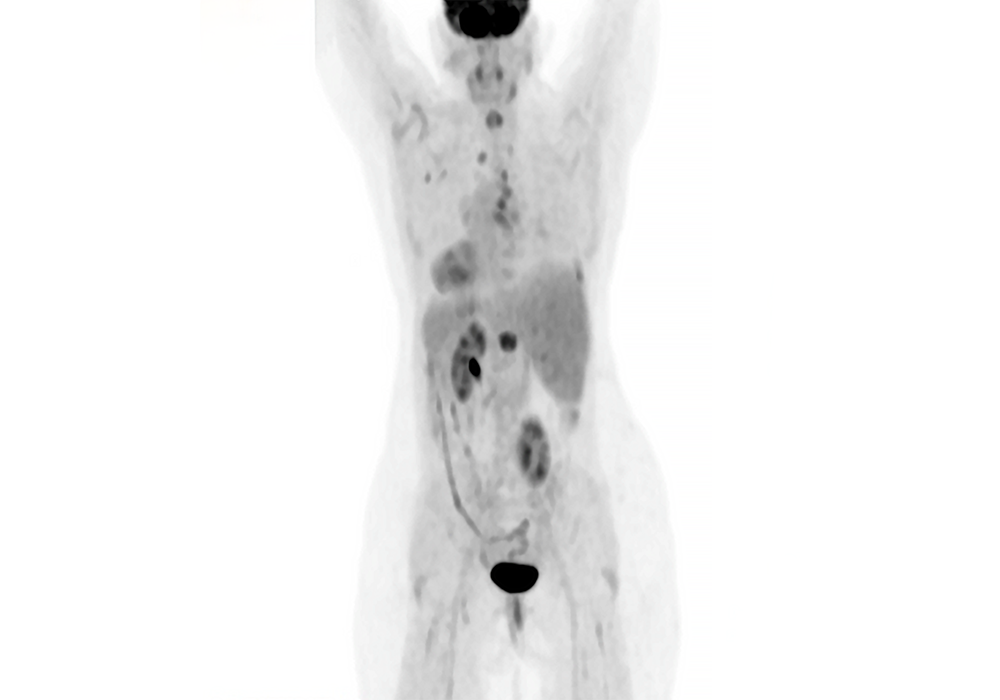
After 3 months of treatment, the patient’s CT scans showed improvement of the infiltrates in the right and left lungs. On the selective RET inhibitor, the patient experienced 18 months of progression-free survival. Additionally, there were no major alterations to his laboratory work that could not have been controlled by adjusting the dose of his pre-existing medications.
You know, there are several key takeaways here. First of all, the judicious use of immunohistochemistry to conserve more tissue for molecular studies, especially in patients with advanced disease. If this is the second, third or fourth biopsy that you're doing in your patient, please notify the pathologist that we don't have to be using a lot of material to prove that this is lung cancer. Another important takeaway is to test it for all actionable biomarkers. I remember we said this at the beginning of the talk. We need to do a comprehensive next-generation sequencing in the tissue or in the blood. But this also has to be done, as I said, in the second, third, or fourth time that the patient fails treatment so we can identify new genetic markers that can be used to rescue the patient. And if we do that, patients will be having targeted agents as treatment. Thank you for listening to this patient case presentation. We hope that the information provided here will have a positive impact in your treatment approach for patients with advanced or metastatic non–small cell lung cancer.
- Perform broad molecular profiling using a validated test to minimize tissue use and potential wastage and assess a for actionable genetic biomarkers
- Have molecular testing results for the actionable oncogenic mutations before starting systemic therapy combined with immunotherapy
- Treat patients with oncogenic driver mutations with targeted first-line therapy for that oncogene
Welcome. My name is Puja Arora. I am a community hematologist-oncologist with University Hospitals Seidman Cancer Center located west of Cleveland at St. John Medical Center. I see most tumor types, and my practice is largely made up of breast, lung and GI malignancies, but I also see malignant and benign hematology.
Today, we’ll be discussing the case of a patient who I initially met as a second opinion. The patient is a female, 53 years of age with stage IV non–small cell lung cancer. When I met this patient, she had already consented to chemoimmunotherapy, but she was seeking a second opinion before beginning treatment. Now, we’ll review the patient’s history leading up to this point after she was transferred to my care.
The patient was previously healthy with no significant past medical history. She was a never smoker and reported no alcohol use. She did report asbestos exposure through her work with a nonprofit organization though could not provide details.She initially presented to the emergency room with shortness of breath to the point she could not walk to her mailbox. A CT of the chest on arrival revealed a soft tissue density in the right side of the mediastinum at the mid-right hemithorax and mediastinal lymph nodes along with a T12 lesion and bilateral pleural effusions. Pathology from thoracentesis revealed adenocarcinoma of pulmonary origin.
After the patient’s initial diagnosis of stage IV non–small cell lung cancer, tissue samples were sent for molecular biomarker testing. A lung cancer hotspot gene panel came back negative for variants in BRAF, EGFR, HER2, KRAS, and MET. Immunohistochemistry of the tissue sample revealed low levels of PD-L1 expression with a tumor proportion score of 11%-20%. The biomarker testing was completed at a well-known outside institution using their in-house assay, which only evaluated 5 of the 9 actionable genomic markers in non–small cell lung cancer. While these 5 markers were negative, it was not comprehensive genomic profiling and, in this case, led to selection of a therapeutic regimen without having all of the necessary information to make that decision.
The patient’s original physician recommended chemoimmunotherapy with carboplatin, pemetrexed and pembrolizumab. Although the patient consented to this treatment plan, she sought a second opinion at my clinic before initiating therapy. Given that the patient was a nonsmoker and did not have the typical risk factors for lung cancer, it was even more important to have comprehensive genomic profiling prior to initiating any therapy.
After reviewing her case and knowing there may be some delays in getting tissue from an outside institution, I elected to obtain a liquid biopsy the day I saw her to allow for a quick turnaround time. Results came back with a RET fusion, which is an actionable biomarker in non–small cell lung cancer.
The patient was now eligible for a targeted therapy, and after some discussion, she decided to move forward with a selective RET inhibitor. She initiated treatment within 1 month of her original diagnosis.
After 3 months of treatment with a selective RET inhibitor, the patient’s PET scan showed resolution of the T12 lesion and pleural effusion. She no longer had any shortness of breath and was able to stop taking all of her pain medications. The patient continues to experience progression-free survival.
Here are some key takeaways from this case. This case highlights why it is so important to have comprehensive genomic profiling before initiating any therapy. Point 3 demonstrates the number of genetic variants that are now available and should be looked for in non–small cell lung cancer. Thank you for listening to this patient case presentation. We hope the information provided here will have a positive impact on your treatment approach for patients with advanced or metastatic non–small cell lung cancer.
The roadmap is designed to be used in discussions HCPs and their patients with NSCLC to facilitate informative and meaningful conversations that will help prepare patients to begin their treatment journeys. Discussion topics include guideline-concordant molecular biomarker testing, as well as recommendations on traditional chemotherapy, chemoimmunotherapy, and oncogene-targeted therapy treatment options for these patients. See the “Related Resources” section to download the roadmap, discussion guide, and accompanying video for your patients.
Every patient diagnosed with non-small cell lung cancer is embarking on a personal treatment journey.
The decisions they make can impact not only their outcomes, but the kinds of obstacles they will face along the way.
While providing medical explanations and trusted advice, you are often your patient’s main source of information. The tone you set and guidance you provide impacts patient decision making.
One way to help patients make the best decision for themselves is to provide as much insight and data as possible. Molecular biomarker testing can provide the most appropriate roadmap to navigate treatment options, but the wait time for results can be difficult.
One way to help patients make the best decision for themselves is to provide as much insight and data as possible. Molecular biomarker testing can provide the most appropriate roadmap to navigate treatment options, but the wait time for results can be difficult.
Do they stay on this route and wait for traffic to clear or do they take an exit and search for a path around the backup?
For a patient, waiting to receive molecular biomarker test results may seem as frustrating as the wait in traffic. But with much higher stakes. When multiple treatment paths can be taken, each requires careful planning for optimal outcomes.
Let’s look at three possible scenarios.
When the dark blue car encounters the traffic jam, they’re anxious and feel the need to begin treatment immediately, instead of waiting for biomarker results.
For the driver, feeling like they need to refuel may lead them to exit and fill up at a nearby gas station before returning to the highway.
Similarly, starting a patient on a round of chemotherapy while waiting for biomarker results may achieve a compromise on treatment path with minimum impact on the efficacy of potential targeted therapies later.
Here, the light blue car also exits the highway to search for an alternate route. To keep moving, they choose a winding path through the city before eventually re-entering the highway later.
However, city streets come with their own obstacles, which can be as frustrating as waiting in the traffic jam and are not necessarily better routes to the driver’s destination.
Similarly, patients may want to explore treatment paths they can begin immediately instead of waiting for biomarker results. For patients eligible for immunotherapy, that treatment route may initially look better because it can be started quickly, but it may make them ineligible for later targeted therapy.
The red car, however, stays on the highway and waits through the traffic jam. It may seem tedious, but sometimes waiting for traffic to clear is the fastest way through.
Although molecular biomarker testing may come with a wait, results provide the most complete roadmap for navigating treatment.
Patients who are positive for an actionable biomarker can be given a targeted therapy, which tend to be better tolerated and have greater efficacy, for a clear path ahead as they navigate their cancer journey.
There are no “right” answers. But your guidance can help patients feel supported as they make difficult decisions.
You can find This Treatment Roadmap and other patient resources to help facilitate discussions with patients about treatment plans by scanning the QR codes.
Managing Disease Progression


Both intrinsic and acquired resistance remains a challenge when patients are treated with TKIs, with most patients experiencing disease progression. Intrinsic mechanisms are present before therapy, whereas acquired mechanisms are induced after therapy is initiated. Common mechanisms of resistance to TKIs include101-104:
- TKI domain or other drug-binding site mutations
- Downstream signaling effector mutations
- Bypass signaling pathways
For patients with a documented prior actionable alteration in whom the disease has progressed with therapy, retesting should be done exclusively on rebiopsy specimens of a progressing lesion. If a biopsy is performed on a suspected bony metastasis, it is critical that decalcification be avoided because some methods of decalcification can preclude any subsequent molecular testing.81
Post-diagnostic Use of Biopsies105

VV-MED-156089
Please rate your satisfaction with the content on the following statements:
Very Dissatisfied
Dissatisfied
Neutral
Satisfied
Very Satisfied