Chronic Lymphocytic Leukemia (CLL)
What is CLL?
Distinction between CLL and SLL1
CLL is the most common type of chronic leukemia characterized by aberrant proliferation of mature monoclonal B cells in the bone marrow. CLL and SLL are denoted as “CLL/SLL” because they are different manifestations of the same disease.1


Median age at diagnosis is 70 years with incidence increasing with age3
More common in men3
Highest incidence in Caucasian population and Western countries1
Genetic basis and can develop in families. First-degree relatives of patients with CLL have double the risk of CLL1
Diagnosis
Patient history and physical examination
Laboratory testing
Histopathology
Immunophenotyping
Test type
|
Diagnostic test
|
Finding necessary for establishing a diagnosis of CLL³,⁴
|
|---|---|---|
| Laboratory testing | Diagnostic test: Complete blood count | Finding necessary for establishing a diagnosis of CLL³,⁴: ≥5000 B lymphocytes/µL in the peripheral blood for at least 3 months |
| Histopathology | Diagnostic test: Blood smear | Finding necessary for establishing a diagnosis of CLL³,⁴: Leukemic cells in blood smear are typically small, mature lymphocytes and have a narrow border of cytoplasm, a dense nucleus without discernible nuclei, and partially aggregated chromatin |
| Immunophenotyping | Diagnostic test: Flow cytometry | Finding necessary for establishing a diagnosis of CLL³,⁴: Confirms clonality of circulating B lymphocytes; usually positive for CD5 antigen and the B-cell markers, CD23 and CD19; weak expression of surface membrane immunoglobulin (CD20 and CD79b) |
For more information on CLL diagnosis, please see CLL slide deck and infographic in the related resources at the bottom of the page.
CLL is usually asymptomatic and discovered via routine blood tests1
Unexplained fevers (>100.5 °F)
Unintentional weight loss (≥10% over 6 months or less)
Night sweats
Early satiety
Fatigue
Swollen lymph nodes
Increased frequency of infections
Autoimmune cytopenia
Enlarged liver or spleen
Risk of progression
Results of evaluating lymphocytosis
Degree of lymph node, spleen, and liver enlargement
Presence of anemia
Presence of thrombocytopenia
CLL-IPI: The CLL-IPI combines genetic, biochemical, and clinical parameters into a prognostic model with 4 risk subgroups: low, intermediate, high, and very high.
prognostication of CLL7,8
Rai Staging System (Primarily United States)9
Stage/risk
|
Characteristics
|
|---|---|
| 0 (Low) | Characteristics: Lymphocytosis, lymphocytes in blood >5 x 10⁹/L clonal B cells and/or >40% lymphocytes in the bone marrow |
| I (Intermediate) | Characteristics: Lymphocytosis with enlarged node(s) |
| II (Intermediate) | Characteristics: Lymphocytosis with splenomegaly, hepatomegaly, or both |
| III (High) | Characteristics: Lymphocytosis with anemia (hemoglobin <11.0 g/dL or hematocrit <33%) |
| IV (High) | Characteristics: Lymphocytosis with thrombocytopenia (platelets <100,000/µL) |
Binet Staging System (Primarily Europe)10
Stage
|
Characteristics
|
|---|---|
| A | Characteristics: <3 areas of lymphoid tissue are enlarged, with no anemia or thrombocytopenia |
| B | Characteristics: ≥3 areas of lymphoid tissue are enlarged, with no anemia or thrombocytopenia |
| C | Characteristics: Anemia (hemoglobin <10 g/dL) and/or thrombocytopenia (platelets <100,000/µL) are present, with any number of enlarged areas |
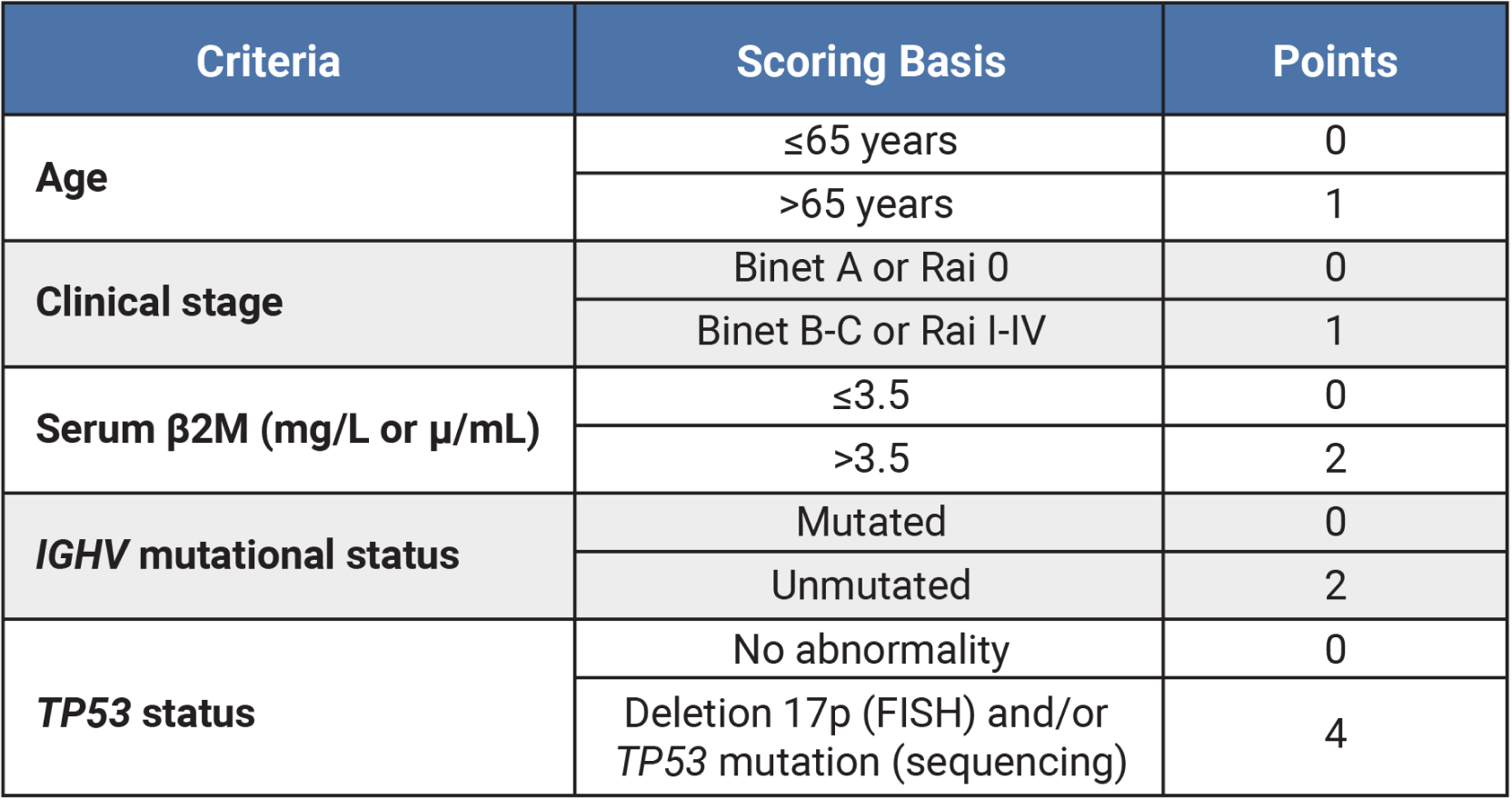
Table outlining CLL-IPI risk group criteria and scoring system based on age (≤65 years = 0 points, >65 years = 1 point), clinical stage (Binet A or Rai 0 = 0 points, Binet B-C or Rai I-IV = 1 point), serum β2M (in mg/L or μ/mL; ≤3.5 = 0 points, >3.5 = 2 points), IGHV mutational status (mutated = 0 points, unmutated = 2 points) and TP53 status (no abnormality = 0 points, deletion 17p [FISH] and/or TP53 mutation [sequencing] = 4 points).
5-year Survival by CLL-IPI Risk Group3,11
CLL-IPI score
|
Risk
|
5-year survival
|
|---|---|---|
| 0-1 | Risk: Low | 5-year survival: 93.2% |
| 2-3 | Risk: Intermediate | 5-year survival: 79.3% |
| 4-6 | Risk: High | 5-year survival: 63.3% |
| 7-10 | Risk: Very High | 5-year survival: 23.3% |
High-risk CLL
High-risk CLL is based on a combination of factors, including genomic biomarkers, as well as patient- and treatment-related risk factors12
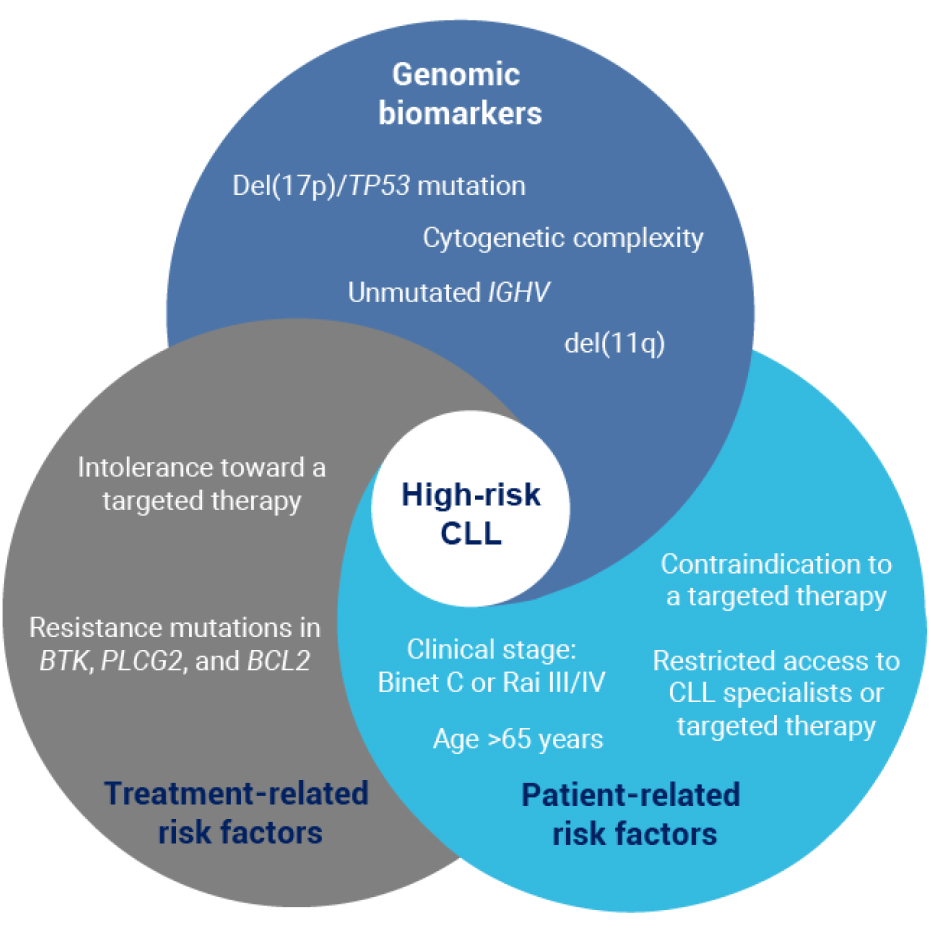
Factors associated with high-risk CLL include genomic biomarkers (del[17p]/TP53 mutation, cytogenetic complexity, unmutated IGHV and del[11q]), treatment-related factors (intolerance toward a targeted therapy and resistance mutations in BTK, PLCG2, and BCL2), and patient-related risk factors (clinical stage: Binet C or Rai III/IV, age >65 years, contraindication to a targeted therapy, and restricted access to CLL specialists or targeted therapy).
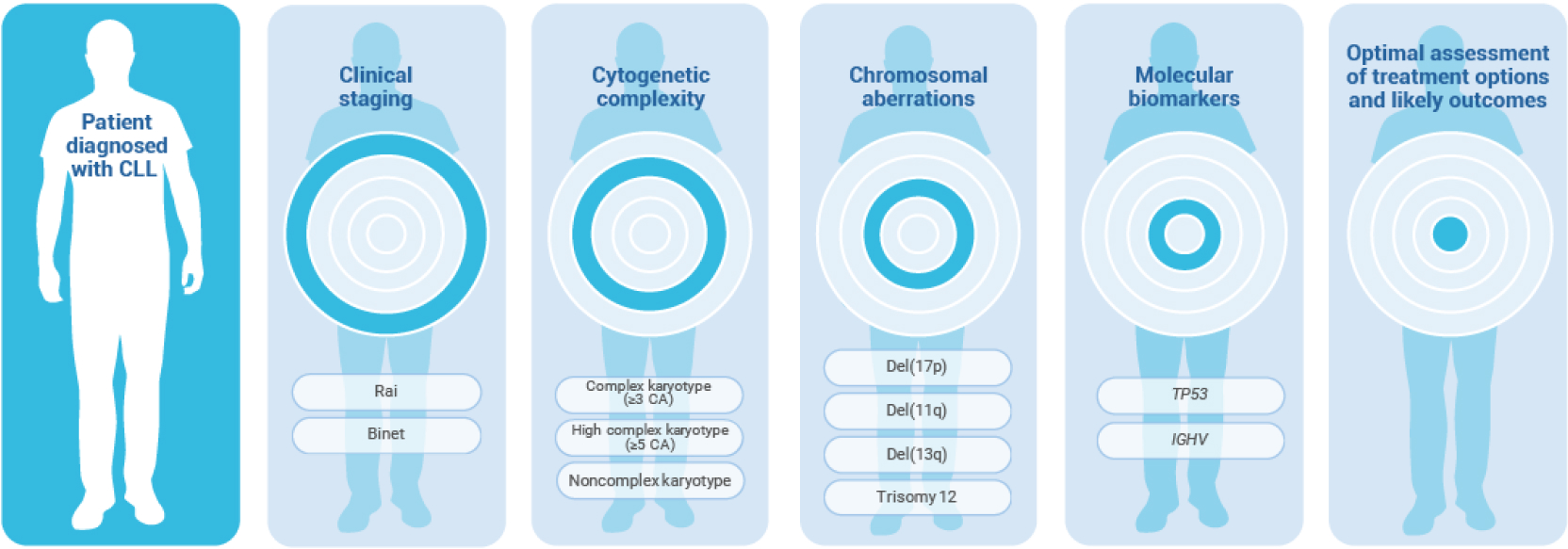
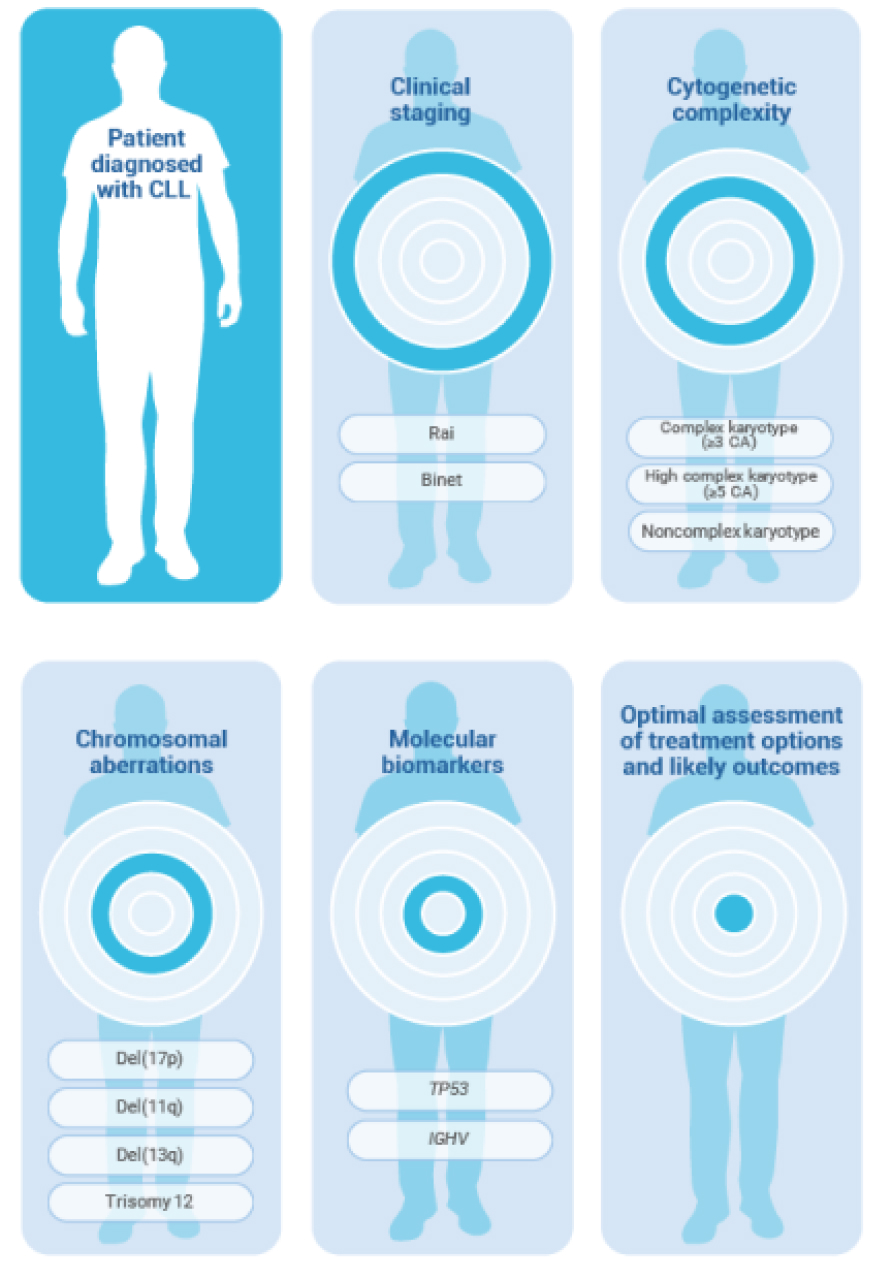
Identifying high-risk CLL in patients diagnosed with CLL involves clinical staging (Rai or Binet system), analysis of cytogenetic complexity (complex karyotype [≥3 CA], high complex karyotype [≥5 CA], or noncomplex karyotype), chromosomal aberrations [including del(17p), del(11q), del(13q) and trisomy 12], and molecular biomarkers (TP53 and/or IGHV), and can help with optimal assessment of treatment options and likely outcomes.
High-risk CLL by clinical staging systems3,7
High-risk CLL—Binet
Anemia (hemoglobin <10 g/dL)
Thrombocytopenia (platelets <100 x 109/L)
Any number of areas of lymphoid tissue enlargement
Referred to as stage C
High-risk CLL—Rai
Lymphocytosis in blood and/or bone marrow
AND
Anemia (hemoglobin <11 g/dL)
OR
Thrombocytopenia (platelets <100 x 109/L)
Referred to as high risk or stage III/IV
Genomic Biomarkers used in CLL prognostication
Biomarker testing is performed at diagnosis to derive prognostic and predictive information from genetic mutations and chromosomal abnormalities associated with CLL, which can inform the treatment plan7
The following biomarkers are associated with poor prognosis in patients with CLL
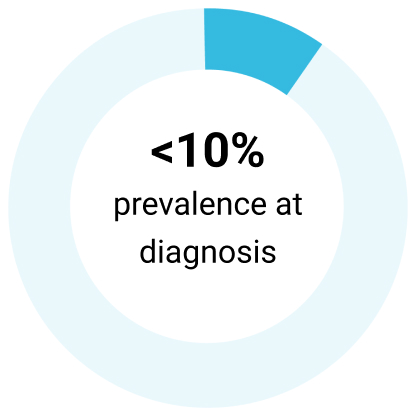
Del(17p)7,15
TP53 mutation8
IGHV unmutated7,14,15
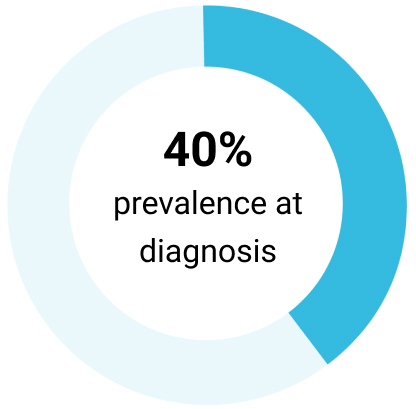
Complex karyotype16
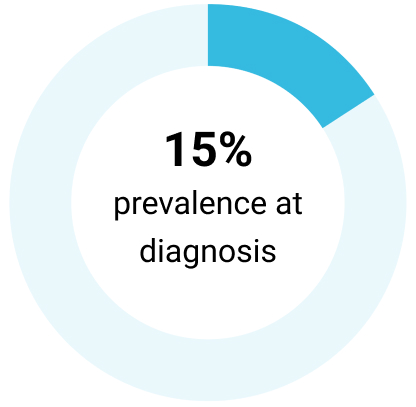
For patients with CLL in which treatment is indicated, the presence or absence of del(17p) and TP53 mutations are most often used to direct treatment selection14

In some cases, acquired resistance during CLL treatment can necessitate additional biomarker testing prior to beginning a new line of therapy17,18
Approximately 80% of patients with CLL carry at least 1 of 4 chromosomal alterations (listed below)3
Chromosomal alteration
|
Description
|
Frequency
|
Risk
|
|---|---|---|---|
| Del(13q) | Description: Critical region of del(13q14) contains miRNAs that regulate apoptosis and cell-cycle progression³,¹⁹ | Frequency: 55% of patients with CLL³ | Risk: Favorable prognosis when alone³,¹⁹ |
| Del(11q) | Description: Frequently encompasses 11q23, which harbors the ATM gene; associated with bulky lymphoma and rapid progression³ | Frequency: 10% of early disease 25% of advanced disease³,ᵃ | Risk: Poor prognosis³,¹⁹ |
| Trisomy 12 | Description: Role in CLL pathogenesis unclear; may be more common in SLL and cases with Richter transformation³,¹⁹ | Frequency: 10%-20% of patients with CLL³ | Risk: Intermediate prognosis³,¹⁹ |
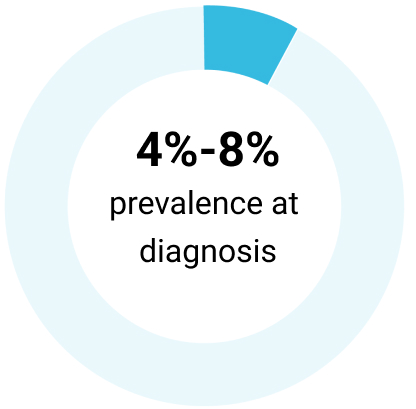
| Del(17p) | Description: 17p13 harbors the TP53 gene, which encodes tumor suppressor protein P53³ | Frequency: 5%-8% of chemotherapy-naïve patients with CLL³ | Risk: Poor prognosis; resistance to genotoxic chemotherapies³ |
aChemotherapy naïve.
References
- Mukkamalla SKR, et al. Chronic lymphocytic leukemia. [Updated 2023 Mar 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470433/
- SEER. Cancer Stat Facts: Leukemia — Chronic Lymphocytic Leukemia (CLL). Accessed August 12, 2024. https://seer.cancer.gov/statfacts/html/clyl.html
- Hallek M, Al-Sawaf O. Am J Hematol. 2021;96(12):1679-1705.
- Hallek M. Am J Hematol. 2019;94(11):1266-1287.
- Kay NE, et al. Blood Rev. 2022;54:100930.
- Lynch DT, et al. Mantle cell lymphoma. [Updated 2023 Jul 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK536985/
- Leukemia & Lymphoma Society. Chronic lymphocytic leukemia. Accessed August 12, 2024. https://www.lls.org/sites/default/files/2023-07/PS34_CLL_Booklet_2023.pdf
- Stefaniuk P, et al. Cancer Manag Res. 2021;13:1459-1476.
- Leukemia & Lymphoma Society. Chronic lymphocytic leukemia. Accessed August 12, 2024. https://www.lls.org/sites/default/files/file_assets/PS34_CLL_Booklet_2019_FINAL.pdf
- Eichhorst B, et al. Ann Oncol. 2021;32(1):23-33.
- International CLL-IPI Working Group. Lancet Oncol. 2016;17(6):779-790.
- Edelmann J, et al. Front Oncol. 2023;13:1106579.
- Martinelli S, et al. Mediterr J Hematol Infect Dis. 2016;8(1):e2016047.
- Campo E, et al. Haematologica. 2018;103(12):1956-1968.
- Yun X et al. Biomark Res. 2020;8:40.
- Baliakas P, et al. Blood. 2019;133(11):1205-1216.
- Shadman M. JAMA. 2023;329(11):918-932.
- Hallek M, et al. Blood. 2018;131(25):2745-2760.
- Lee J, Wang YL. J Mol Diagn. 2020;22(9):1114-1125.
VV-MED-163244
Please rate your satisfaction with the content on the following statements:
Very Dissatisfied
Dissatisfied
Neutral
Satisfied
Very Satisfied